Have you ever dreamt of an ingenious medical device that revolutionizes patient care? Perhaps it’s a tool that streamlines diagnosis, a prosthetic that enhances mobility, or a treatment that delivers unparalleled results.
But the path from a brilliant idea to a life-changing reality can be daunting. Medical device development is a complex dance between cutting-edge technology, rigorous regulations, and a deep understanding of unmet needs. Yet, within this intricate process lies the immense potential to improve countless lives.
This guide unveils the secrets to navigating medical device development, empowering you to transform your vision into a tangible force for good.
As you embark on this exciting journey, remember that IntelliSoft, a medical device development company, stands by your side. With over 15 years of experience in the healthcare industry, we possess the expertise to guide you through every step of the medical device development process.
From brainstorming sessions to regulatory hurdles, our team is dedicated to helping your groundbreaking app reach the patients who most need it.
Table of Contents
ISO Standards for Medical Device Development
Imagine a world where medical devices, from the sleekest diagnostic tools to the most intricate prosthetics, emerge not from chance but from a meticulously designed process. A process that prioritizes patient safety, optimizes functionality, and streamlines development – that’s the power of ISO standards.
The International Organization for Standardization (ISO), a global, independent body, sets the stage for excellence with these voluntary standards. Two crucial ones stand out in the realm of medical devices: ISO 13485 and ISO 14971.
ISO 13485: Your Quality Management Compass
Ever feel lost in the labyrinth of cardiac medical device development? ISO 13485, established in 1996 and revised in 2016, acts as your compass. It outlines the roadmap for a robust Quality Management System (QMS). Think of a QMS as the organized filing cabinet for all your crucial processes and procedures – from initial design to bustling production. This includes design controls, ensuring your brilliant idea translates into a safe and effective appliance.
ISO 14971: Taming the Risk Beast
Let’s face it; medical device design and development involve some inherent risk. ISO 14971, initially released in 2007 with a 2019 update, empowers manufacturers with a risk management in medical device development methodology. This isn’t about eliminating risk (that’s a superhero’s job) – it’s about identifying potential hazards, assessing their severity, and implementing controls to minimize them. By integrating risk management into your QMS (as encouraged by ISO 13485), you can proactively safeguard patient well-being.
A successful medical device product development process requires a holistic approach. Here are some additional questions to consider:
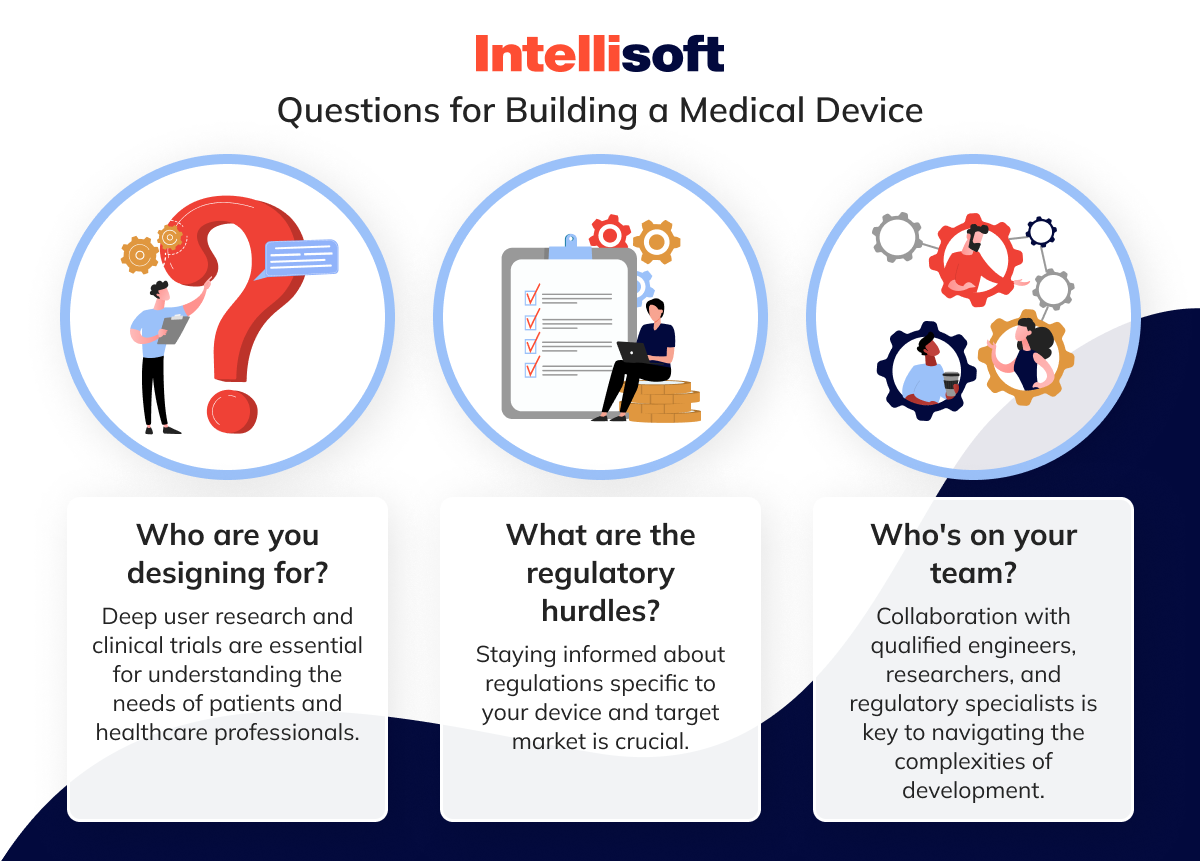
By embracing a comprehensive approach to the medical device software development services incorporating ISO standards alongside these other crucial elements, you can significantly increase your chances of bringing a safe, effective, and marketable medical device to the world. Remember, this isn’t just about building an appliance — it’s about building a better future for patient care.
Stages of Processing Medical Devices
The world of medical device product development is a marathon, not a sprint. While your vision might be burning bright, rushing through the process can be a recipe for disaster. Studies show that a staggering 75% of medical device startups fail. But fear not; this guide will unveil the key to navigating this complex landscape and avoiding those pitfalls.
The world of development medical device regulations can feel like a jungle gym with tangled rules. Every new appliance needs to meet specific requirements or prove it’s practically identical to an already approved product. The good news? Regulatory agencies like the FDA and the EU are working together to streamline the process. This means less confusion and more focus on patient safety, which is the ultimate goal.
The specific regulations you encounter depend on your device’s risk level. Think of it like a spectrum – low-risk appliances have a smoother path compared to high-risk ones. Here’s a breakdown of the approval pathways based on risk classification:
FDA Classification System
- Class I (Low Risk). These devices typically have a simpler approval process, often following the 510(k) pathway. This pathway focuses on demonstrating that your device is substantially equivalent to a predicate appliance already on the market.
- Class II (Moderate Risk). These appliances may require more rigorous testing and data compared to Class I devices. They may still qualify for the 510(k) pathway, but some may require the Premarket Approval (PMA) pathway, depending on the specific risk profile.
- Class III (High Risk). These appliances pose the highest potential risk and require the most stringent approval process – the Premarket Approval (PMA) pathway. This pathway demands a significant amount of data to validate the device’s safety and efficacy.
EU MDR Classification System
The EU MDR utilizes a system with four appliance categories and five risk classifications. The assigned class will determine the depth and amount of data required for approval.
- Class I (Low Risk). This class is further divided based on sterility and measuring function (non-sterile or no measuring function, sterile and measuring function).
- Class IIa, Class IIb, and Class III. These classes represent increasing levels of risk (medium, medium/high, and high respectively).
Let’s take a closer look at the 5 phases of medical device development and processing.
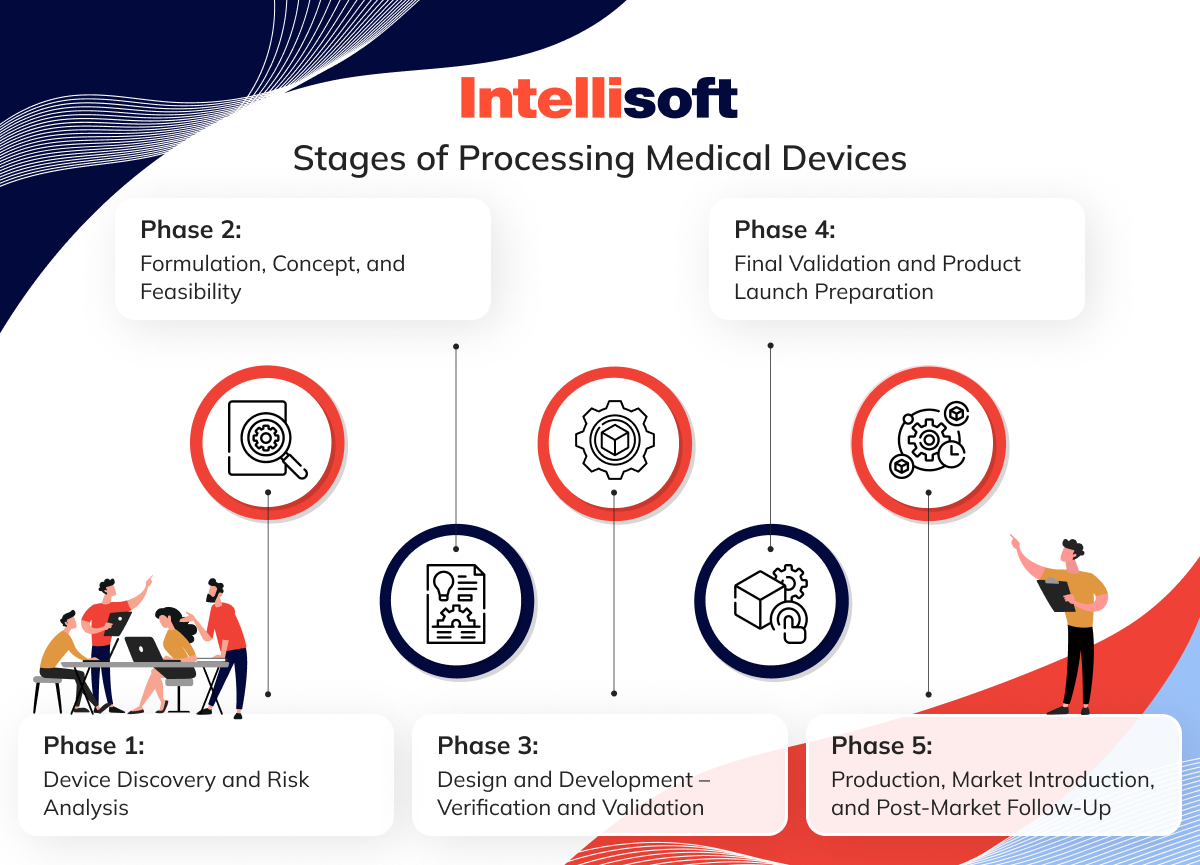
Phase 1: Device Discovery and Risk Analysis
This is the foundation upon which your entire project is built. Here, you’ll take a deep dive to:
- Identify the unmet need. What problem are you trying to solve with your medical device app development? Conduct thorough user research to understand the specific challenges faced by patients, doctors, or healthcare institutions.
- Define your target audience. Who will benefit most from your appliance? Tailoring your solution to a specific group ensures it addresses their unique needs.
- Evaluate the competitive landscape. Who are your competitors, and what are their strengths and weaknesses? This analysis helps you identify opportunities for differentiation.
- Scrutinize feasibility. Can your appliance be realistically manufactured within budget and time constraints? Is the technology mature enough to support your vision?
- Craft a comprehensive risk assessment. Proactive identification of potential hazards associated with your device is paramount.
Documenting your plan is crucial at this stage. By answering questions like “Why is there a need for this product?” and “Do I have the resources to develop this idea?” you’ll gain clarity and direction for the journey ahead.
Phase 2: Formulation, Concept, and Feasibility
Phase 2 transforms your innovative idea into a tangible concept. Here, you’ll:
- Develop a detailed product specification. This document outlines the technical requirements and functionalities of your appliance, serving as a blueprint for development.
- Craft a compelling concept design. This could involve sketches, 3D models, or even physical prototypes to visualize your device and gather user feedback.
- Refine your risk assessment. As your concept evolves, so too should your understanding of potential risks. Continuously update your risk management plan to mitigate potential issues.
- Secure funding. Depending on the complexity of your appliance, additional funding may be necessary for prototyping and testing.
- Embrace the voice of the customer. Solicit feedback through surveys, competitor analysis, and market research to ensure your design truly addresses user needs.
Phase 2 should culminate in a feasible concept, a validated technical approach, and a continuously evolving risk management plan. This solid foundation sets the stage for the detailed design and development phase.
Phase 3: Design and Development – Verification and Validation
Now, it’s time to bring your concept to life. Phase 3 focuses on:
- Detailed engineering design. This stage involves creating precise blueprints and technical drawings that define your appliance down to the finest detail.
- Prototyping and iterative refinement. Functional prototypes allow for rigorous testing, user feedback, and ongoing design improvements.
- Verification and Validation. Verification ensures your device is built according to specifications. Validation confirms it performs as intended and meets user needs.
- Rigorous testing. Your device will undergo a battery of tests to ensure safety, efficacy, and compliance with relevant standards.
By the end of Phase 3, you’ll have a fully functional prototype validated for performance and a comprehensive understanding of its capabilities and limitations. This is a significant milestone, marking a crucial step closer to bringing your innovation to market.
Phase 4: Final Validation and Product Launch Preparation
The finish line is in sight. Phase 4 focuses on:
- Final risk management. Addressing any remaining risks identified during testing and ensuring a robust risk mitigation plan is in place.
- Regulatory submissions. Navigating the approval process with the FDA or EU MDR involves compiling technical documentation and preparing for potential audits.
- Manufacturing scale-up. Transitioning from prototypes to a defined manufacturing process ensures consistent high-volume production quality.
- Marketing and sales strategy development. Creating a plan to bring your appliance to market, secure customer adoption, and effectively communicate its value proposition.
Gathering evidence to support your regulatory submissions is crucial in this phase. Data from biocompatibility, electrical safety, and stability testing will all be required.
Phase 5: Production, Market Introduction, and Post-Market Follow-Up
Congratulations, your product development medical device is ready to positively impact the world. Phase 5 focuses on:
Full-scale production. Manufacturing your device at scale by established quality control procedures. This ensures consistent quality and minimizes the risk of defects.
Market launch. Executing your marketing and sales strategy to reach your target audience and drive product adoption. This might involve activities such as attending trade shows, developing targeted marketing campaigns, and establishing distribution channels.
Post-market surveillance. Continuously monitoring your appliance’s performance in the real world to identify and address any emerging issues. This involves collecting data from customer feedback, analyzing it and identifying trends and patterns, and taking corrective action.
Building a strong customer service system is also crucial in Phase 5. By addressing customer concerns promptly and effectively, you can build trust and ensure the long-term success of your medical device.
Remember, the medical device development process doesn’t end here. By diligently following these five phases and continuously learning from your experience, you can significantly increase your chances of bringing a safe, effective, and marketable medical appliance to market, ultimately improving lives and revolutionizing healthcare, and a lot of medical device software development companies are ready to assist you in that.
Related Readings:
- App for Doctor Appointment Development: A Comprehensive Guide
- Transforming Healthcare Communication: Integration of HL7 Interface Engine
- Prescription Management Software: The Future of Pharmacy
- Hospital App Development: Innovate, Integrate, and Provide Better Care
- Automation in the Industry of Healthcare: Main Benefits for Business
5 Key Activities to Be Ready for Market Entry in Healthcare
The journey from a brilliant medical appliance idea to a product making a real difference requires meticulous medical device research and development planning. Here are 5 key activities and medical device development services to ensure your successful market entry in the healthcare industry:

Market Research
Medical device development from prototype to regulatory approval starts with market research. A successful medical appliance solves a real problem. Conduct thorough market research to pinpoint the unmet needs of your target audience, whether it’s patients, doctors, or healthcare institutions. Analyze your competition to understand their strengths and weaknesses, and assess the market size and potential to determine if there’s a sufficient demand for your appliance.
Cost and Timeframe Considerations
Medical device product development is an investment. Develop a comprehensive budget that factors in research, development, regulatory approvals, manufacturing, and marketing costs. Create a realistic timeline that acknowledges the marathon nature of this process, considering potential delays due to regulatory hurdles. Depending on the complexity of your appliance, securing additional funding from investors, grants, or loans may be necessary.
Identification of Medical Device Class for the FDA
The next step of the medical device product development lifecycle is understanding the regulatory landscape. Medical appliances are categorized based on their level of risk. Classify your medical device product development certification according to the FDA classification system (or EU MDR if applicable) to determine the appropriate regulatory pathway and approval process. Compile the necessary technical documentation, risk assessments, and other information for regulatory submissions, and be prepared for potential audits by regulatory bodies verifying your compliance with quality standards.
Final Validation of the Medical Device
Safety and efficacy are paramount. Your appliance must undergo rigorous testing through specific testing and medical device development tools to ensure it meets relevant standards. Refine your design based on test results, optimizing performance and addressing any identified issues. Ultimately, you need to demonstrate that your device meets all intended functionalities and performs as expected.
Preparation for Medical Device Launch
A successful launch requires a well-defined strategy. Develop a marketing and sales plan either on your own or with the help of medical device product development companies to reach your target audience, secure customer adoption, and effectively communicate the value proposition of your device. Establish distribution channels to get your appliance into the hands of healthcare providers and ultimately, the patients who need it. Finally, medical device development firms build a strong customer service system to foster trust and ensure the long-term success of your appliance.
How Long Does It Take to Develop a Medical Device?
Bringing a medical appliance from concept to reality is no small feat. Imagine a winding path, filled with challenges and milestones, leading to a product that can revolutionize healthcare. The time it takes to follow this path depends heavily on the classification of your device, as determined by regulatory bodies like the FDA. Let’s explore the average medical device development timeline.
Class 1 Devices (Low-Risk)
These devices pose the lowest potential risk to patients and typically have a faster development timeline. Think of thermometers, bandages, or non-invasive blood pressure cuffs. Obtaining approval for a Class 1 device through the 510(k) pathway often takes 12-18 months. This streamlined process involves demonstrating that your appliance is substantially equivalent (similar in design and function) to an already approved predicate appliance.
A 510(k) is essentially a premarket notification submitted to the FDA that details how your appliance compares to a substantially equivalent appliance that is already on the market.
Class 2 Devices (Moderate-Risk)
Falling into the medium-risk category are devices like powered wheelchairs, nebulizers, and some catheters. The approval process for Class 2 devices can be more rigorous than Class 1, and the timeline can range from 24 to 36 months. The 510(k) pathway may still be an option, but some Class 2 devices may require the Premarket Approval (PMA) pathway, involving more extensive testing and data collection to demonstrate safety and efficacy.
Class 3 Devices (High-Risk)
These devices pose the highest potential risk and require the most stringent approval process. Think of pacemakers, artificial heart valves, or certain implants. The development timeline for Class 3 devices can be lengthy, often taking 5 years or more. The PMA pathway is mandatory for Class 3 devices, demanding a significant amount of clinical data to validate the device’s safety and effectiveness.
Challenges and Opportunities in Medical Device Development
Medical device product development life cycle is a complex but rewarding endeavor. While it offers the chance to significantly improve patient outcomes, it also presents significant hurdles to overcome. Let’s explore some of the key challenges and opportunities in this field.

Cost and Funding
Bringing a medical appliance to the market requires substantial financial investment. Research, development, regulatory approvals, manufacturing, and marketing all contribute to the high cost. Securing sufficient funding through venture capital, grants, or strategic partnerships is crucial for navigating this financial landscape.
Regulatory Complexities
Regulatory bodies like the FDA impose stringent requirements to ensure patient safety and device efficacy. Meeting these requirements can be challenging, but successfully navigating the regulatory process builds trust with healthcare professionals and patients by demonstrating your commitment to a safe and effective product.
Talent and Expertise Shortage
Medical device development life cycle requires a unique blend of expertise in engineering, design, regulatory affairs, and clinical research. Finding and retaining skilled professionals in this specialized field can be difficult. However, fostering a collaborative environment that attracts and retains top talent can lead to a more innovative and efficient development process.
Emerging Technologies and Changing Market Dynamics
The medical device business development landscape is constantly evolving. Artificial intelligence, big data, and robotics present exciting possibilities for innovation. The challenge lies in staying current with these advancements and integrating them into your appliance design. Embracing these emerging technologies presents a tremendous opportunity to create truly groundbreaking solutions that address unmet medical needs and revolutionize healthcare delivery.
Banner
Text: Hire Healthcare Software Developers
CTA: Learn more
Link: https://intellisoft.io/healthcare-software-development-services/
Conclusion
Navigating the complex world of medical appliance development is undeniably challenging, but with the right approach, it is immensely rewarding. By adhering to ISO standards, thoroughly understanding regulatory pathways, and meticulously planning each development phase, you can significantly increase your chances of bringing a groundbreaking device to market. Remember, this journey is not just about innovation; it’s about improving patient care and building a legacy of quality and safety.
As you venture into the development of your medical appliance, the support and guidance of an experienced medical device development companies can make all the difference. With over 15 years of experience in the healthcare industry, IntelliSoft is equipped to help you at every stage, from initial brainstorming to navigating regulatory hurdles. Our dedicated team is committed to ensuring your innovative appliance reaches the patients who need it most. Contact IntelliSoft today to learn how we can assist you in transforming your visionary idea into a life-changing reality and receive medical device development consultancy UK.