The medical software industry is poised for a remarkable surge, expected to balloon from $19 billion in 2022 to $167.59 billion by 2032. This growth represents a golden opportunity for those eager to develop software for medical devices. However, it’s important to recognize that success in this field requires more than exceptional coding skills; a deep understanding of medical standards and regulations is also essential.
This guide is your blueprint for success. It will walk you through every stage of the medical device software development process—from the initial spark of an idea to the successful launch of your product. Along the way, you’ll discover how to tackle complex regulations, create user-friendly designs, and leverage the latest technology.
So, are you ready to begin? Let’s jump in.
Table of Contents
What is Medical Device Software?
Medical Device Software (MDSW) represents a groundbreaking leap forward in healthcare. It is designed to independently carry out medical tasks without relying on traditional hardware. It stands at the cutting edge of contemporary medicine, significantly influencing diagnostics, treatment, and patient monitoring.
As the healthcare landscape evolves, MDSW is rapidly becoming a vital component. It powers various medical devices, from life-saving pacemakers and insulin pumps to cutting-edge surgical robots and advanced imaging systems. MDSW’s impact on healthcare is profound, bringing benefits such as:
- Real-time feedback to healthcare providers
- Automation of tasks, thereby minimizing the risk of human error
- Personalized and targeted treatment options
- Improved accessibility and affordability of medical care
Consider a heart rate monitoring app on a wearable device as an example. This software gathers and analyzes heart rate data and presents it in a user-friendly format. More crucially, it can notify medical professionals if abnormalities are detected, making it an invaluable tool for continuous diagnosis and patient monitoring.
Medical device software and app development is a meticulous endeavor that focuses on developing a digital product specifically for medical applications. This process entails careful design, development, testing, and deployment, all while complying with stringent regulatory standards to ensure safety and effectiveness.
“The challenge in medical device software development is ensuring that the code is not only correct, but also safe, reliable, and compliant with stringent regulatory requirements.” — David Vogel, Principal Consultant at Intertech Engineering Associates
Developing software for medical devices demands a careful blend of medical expertise and technological innovation. Strict regulatory requirements and quality standards govern the process, aiming to create reliable, accurate, and user-friendly digital products to help healthcare providers deliver better patient care.
A recent study found that around 83% of medical IoT devices in the United States operate on outdated or unsupported software systems. These legacy systems, often abandoned by their medical device software development companies, pose significant security risks, compromising nearly 98% of sensitive medical data traffic or inadequately encrypting it.
The widespread use of outdated software in healthcare highlights an urgent need for modernization. Upgrading these systems is essential to protect sensitive medical data and to ensure that software remains reliable, accurate, and secure. As technology evolves and becomes more integrated into medical devices, adhering to regulatory standards and developing innovative, safe, and compliant digital products is more important than ever.
Reliable and advanced medical device software is key to better patient care, more accurate diagnoses, and more efficient healthcare delivery, ultimately leading to higher quality and more effective medical services.
Software as a Medical Device (SaMD) Market Size
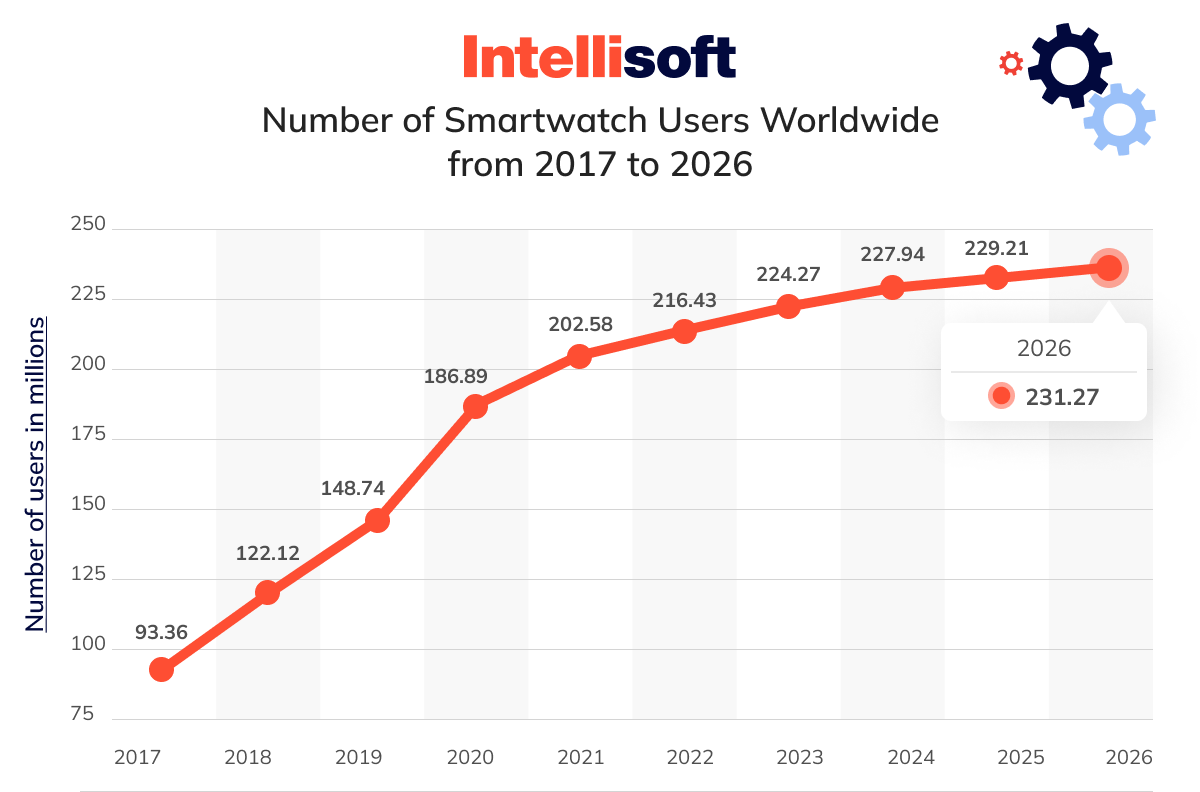
The Software as a Medical Device (SaMD) market is swiftly emerging as one of the most vibrant segments in the MedTech industry. With the number of SaMD companies growing faster than any other area of medical software, this market is on track for impressive expansion. By 2027, it’s expected to reach a staggering $8.2 billion, reflecting a robust annual growth rate of 11.0%.
Several factors drive this rapid growth, including increasing patient demand and the expanding application of SaMD in areas such as diagnosis, treatment, and patient monitoring. Incorporating AI and machine learning further propels these devices’ capabilities, making them more effective and versatile. Additionally, the surge in SaMD applications within smartwatches brings this technology closer to the everyday consumer, significantly enhancing its appeal and accessibility.
By 2027, the Desktop/Laptop segment is expected to lead the Software as a Medical Device (SaMD) market. North America currently holds the largest market share, a trend that shows no sign of shifting. Given the increasing importance of this market across various stakeholders, investing in Software as a Medical Device development presents a significant opportunity that should not be overlooked.
Types of Medical Device Software
Medical device software comes in two primary forms: Embedded Medical Systems and Software as a Medical Device (SaMD). Let’s explore each of these categories and why they matter.
Embedded Medical System Software (EMSSW)
Embedded Medical System Software (EMSSW) is the brain behind many medical devices, ensuring they function smoothly and gather real-time data. Imagine the software in a defibrillator, for example—it controls the precise timing and delivery of electrical shocks to jumpstart the heart during cardiac arrest.
Another critical example is the software in insulin pumps, which carefully manages insulin delivery to stabilize blood sugar levels for diabetic patients. This type of software is often coded in languages such as C, C++, Java, and Python, ensuring these life-saving devices do their job accurately and reliably.
Software as a Medical Device (SaMD)
Software as a Medical Device (SaMD) is a game-changer in healthcare, offering powerful, standalone software designed specifically for medical applications. Unlike traditional embedded software tied to specific hardware, SaMD operates independently, running seamlessly on PCs, mobile devices, and other platforms. Imagine mobile health apps that keep track of your vital signs or advanced diagnostic tools that analyze medical images—these are all examples of SaMD in action.
The technology behind SaMD varies depending on the platform. Swift or Java is often used for mobile apps, while JavaScript, Python, or Java are popular choices for web-based applications.
Both embedded medical software (EMSSW) and SaMD play vital roles. EMSSW drives the core functionality of medical devices, while SaMD offers innovative, standalone solutions that enhance diagnosis, treatment, and overall patient care.
Who May Need Software Development for Medical Devices?
MedTech software development is booming, with companies of all sizes—from established medical device manufacturers to innovative Healthtech startups—racing to stay ahead. These companies are constantly looking for medical device software development services, whether it’s to power advanced medical devices, develop cutting-edge medical apps, or provide end-to-end software solutions. By tapping into these capabilities, they can enhance their products, deliver better patient outcomes, and maintain a competitive edge in the rapidly evolving industry.

Benefits of Medical Device Software Development
Integrating advanced digital products into medical devices makes healthcare services more efficient and reliable, ultimately enhancing patient safety. Here are some key advantages of developing medical software:
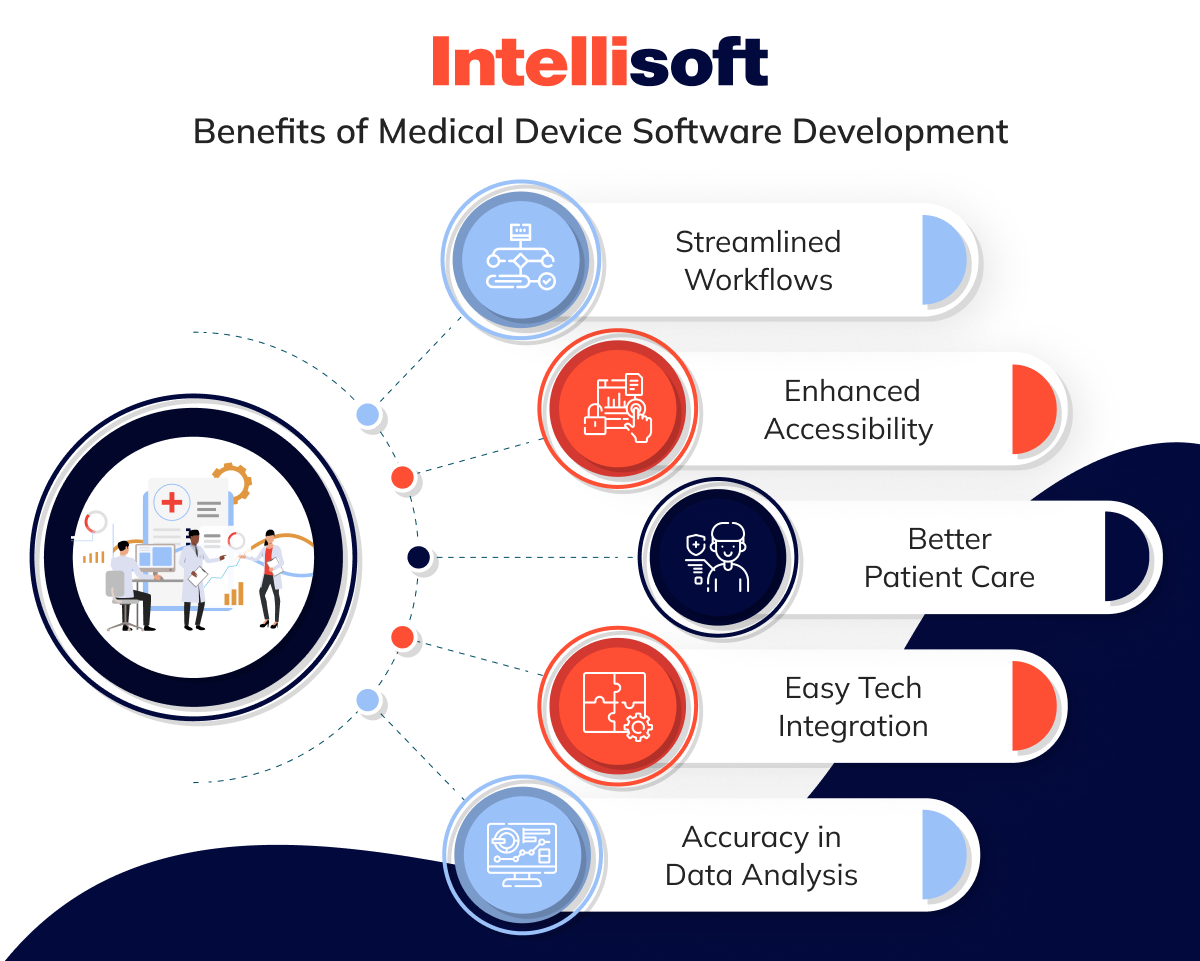
Streamlined Workflows
Through seamless medical device software engineering, you can design and integrate solutions that streamline operational workflows and boost productivity in healthcare. These advancements enable businesses in the sector to shift their focus away from administrative tasks, directing more attention toward patient care.
Enhanced Accessibility
Agile medical device software development extends healthcare services beyond traditional clinical settings, enhancing accessibility for patients who need quick, affordable, and convenient medical care.
Better Patient Care
One key advantage of investing in medical device development software is its ability to deliver precise diagnostics, tailored treatment plans, and advanced monitoring, all of which contribute to better patient care.
Easy Tech Integration
Adopting advanced technologies such as AI development, IoT integration, and cloud computing in healthcare can significantly enhance the performance and functionality of medical devices. This method allows medical institutions to deliver more effective healthcare solutions and better patient care.
Accuracy in Data Analysis
Software as a Medical Device (SaMD) enhances data classification and analysis, enabling healthcare businesses to extract actionable insights. This capability is crucial for making informed decisions and staying competitive.
Related articles:
- Best Examples Of Successful Healthcare IT Start-Ups From Denmark
- Big Data Analytics in Healthcare: Role and Benefits
- Data Warehousing in Healthcare: Transformative Strategies
- Hospital Management Software: Drive Superior Patient Outcomes
- What is EHR Software? Build Your Custom System and Digitize Doctor’s Scribbles with IntelliSoft
How to Build Custom Medical Device Software?
Navigating the development process for custom medical device software requires meticulous attention to several key steps. Each phase is crucial to ensuring compliance with regulatory standards, precision, and operational excellence, which are needed for high-stakes digital products. Let’s explore these essential steps in more detail.
Understand Your Regulatory Requirements
Embarking on the medical device software development process requires navigating a complex web of regulatory standards set by authorities like the FDA, HIPAA, and other local bodies. These regulations aren’t mere formalities; they serve as critical safeguards, ensuring that your software meets the highest safety, reliability, and quality standards.
Adhering to these regulations goes beyond simply achieving market approval. It’s about protecting patient well-being, with these standards guiding every phase of medical device software development—from outlining your software’s purpose to embedding essential features and safety protocols.
Market Research and User Requirement Clarification
Thorough market research is vital for developing an effective and user-friendly medical device app. It provides a deep understanding of the healthcare environment, current industry trends, and users’ needs. Engaging with healthcare professionals, patients, and stakeholders helps pinpoint specific user requirements and preferences.
These insights define the app’s features, design, and functionality. By tailoring the final product to address the precise needs of its users, we can ensure it is both effective and easy to use.
Find an Experienced MDSW Development Firm
Partnering with a custom medical device software development firm with a strong reputation and extensive experience is essential. Equally important is the firm’s proven history of adhering to regulatory requirements.
A trusted company such as IntelliSoft excels in software development and offers valuable insights and guidance. Their expertise is critical in ensuring compliance, creating robust, high-quality digital products, and providing innovative solutions.
Planning Stage with Your Hired Development Company
Now is the ideal time to partner with your chosen medical device software development company and start project planning. This phase involves defining the project’s scope, establishing clear milestones, and developing a robust strategy to guide the process.
Effective planning is key to building a strong foundation, ensuring everyone is aligned, and matching the development process with your specific goals and timelines. With careful planning, you can navigate the project efficiently, avoid unnecessary delays, and confidently reach your objectives.
Development and Integration
Once the groundwork has been laid, the medical device software development process starts with a strong focus on quality, precision, and adherence to regulatory standards. During this critical phase, the digital product must be designed to integrate effortlessly within the healthcare infrastructure, ensuring smooth data exchange across various systems.
Implementation and Monitoring
Once the software is ready for implementation in the healthcare environment, this phase in developing medical device software requires meticulous monitoring and evaluation of its performance. Continuous oversight throughout the development process is essential to detect potential issues or areas needing refinement. This approach ensures the software operates efficiently and effectively, safeguarding its functionality.
Support and Maintenance
After implementation, ongoing support and maintenance are vital to ensuring the software remains reliable and secure and performs optimally in the healthcare environment. This stage in the medical device software development life cycle involves addressing emerging issues, applying updates or patches, and maintaining the software’s compliance with industry standards. Continuous support and maintenance are key to sustaining the efficiency of medical device software.
Tech Stack to Use in Medical Device Software Development
A wide array of technical solutions and tools are available to develop digital products for medical equipment. The specific configuration you’ll need will depend mainly on the scope and details of your project, something best determined in consultation with your vendor. If you’re interested in discussing particular tech options or the overall process of medical device software development, we encourage you to contact one of our experts.
Typically, the tech stack employed in medical device software engineering might include a combination of the following:

- Integrated Development Environments (IDEs) and compilers
- Embedded coding languages: C, C++, Python, MicroPython, and Java
- Debugging tools and software
- Emulators
- Testing software and devices
- Cloud development platforms: Azure, AWS, Digital Ocean, and Google
- Integrated solutions: Wearables, mobile, and IoT devices
- Front-end development: Angular, React, Vue, Node.js, and Core JavaScript
- Supportive software: C#, Node.js, JavaScript, TypeScript, Dart, PHP, and SQL
- Middleware and additional third-party modules: 3D imaging, 3D printing, AI, ML, AR/VR, and medical SaaS
“Medical device software development isn’t just about creating functionality—it’s about creating trust and ensuring that every line of code contributes to patient safety.” — Brian Shoemaker, Ph.D., Principal Consultant at Shoebar Associates
Factors to Consider When Developing Software for Medical Devices
Developing software for the medical industry requires meticulous planning, especially concerning regulatory compliance and data security.
Regulatory Requirements and Quality Standards
Strict compliance with medical device software development standards is crucial when designing a digital product for medical devices, guaranteeing patient safety and product effectiveness.
Here are some standard regulatory requirements by region:
- United States. FDA, HIPAA, HITECH
- European Union. EMA, MDR, GDPR, CE
- Canada. PIPEDA, CMDR
- Australia. TGA, Privacy Act
- United Kingdom. MHRA, Data Protection Act
- International standards. IMDRF and ISO
Data Security and Privacy
Data security is crucial in the medical device software industry due to the sensitive nature of the information involved. Protecting patient data requires several vital practices:
- Encryption. Employing encryption techniques to ensure data confidentiality.
- Access control. Implementing mechanisms to regulate who can access data.
- Auditing and logs. Keeping detailed logs to monitor system activities for security purposes.
- Disaster recovery. Establishing regular backups and recovery plans to mitigate data loss.
How to Choose the Right Development Partner
Whether starting from scratch with your medical device software or needing extra hands to boost an ongoing project, finding the right developers is crucial—and not always easy. Here are some essential tips to help you build a capable team for your project:
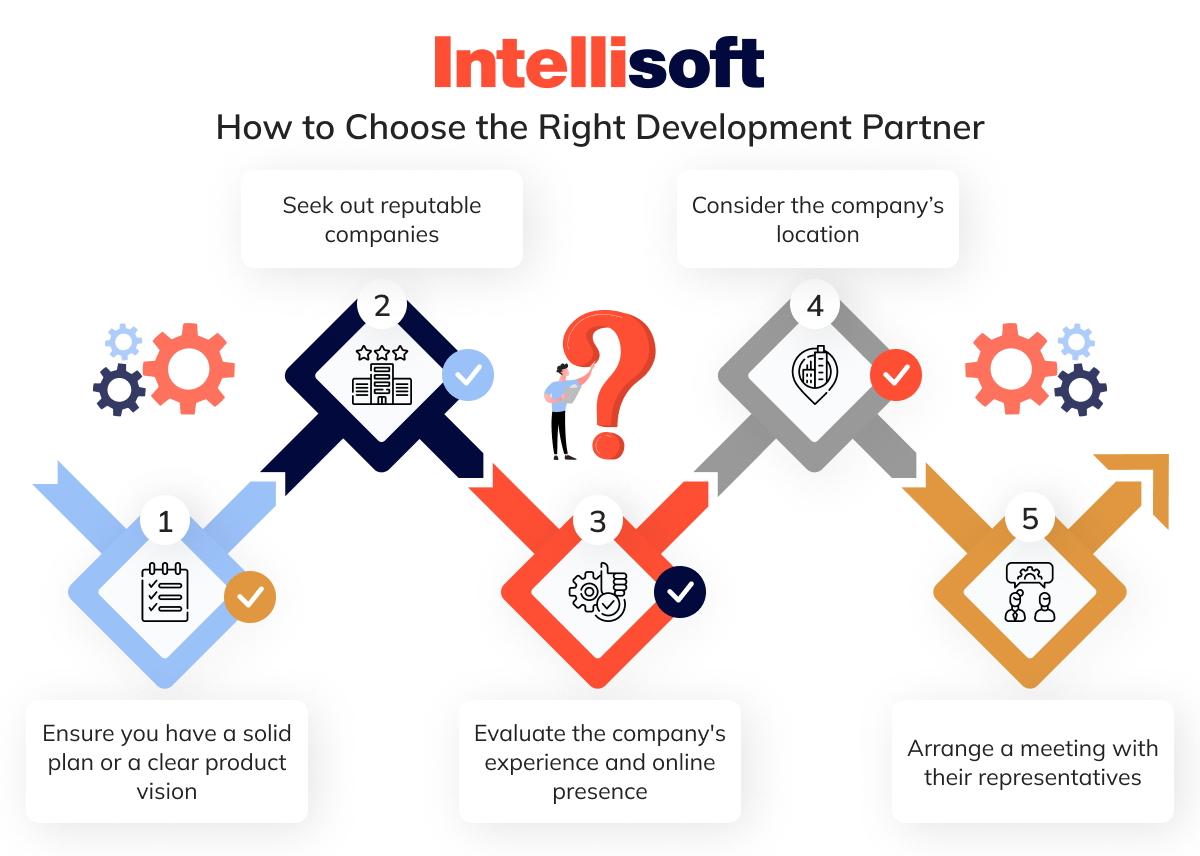
First, ensure you have a solid plan or a clear product vision. A well-thought-out software development plan medical device or vision can save significant time when collaborating with software experts.
Second, seek out reputable companies. Take the time to read their testimonials and reviews across various directories, such as Clutch and GoodFirms.
Third, evaluate the company’s experience and online presence. Do they have credible case studies in health technology, and are there genuine customer reviews on their website?
Next, consider the company’s location. Are they HIPAA-compliant? Do they operate from a legitimate office or function as a virtual agency hiring developers from freelance platforms?
Finally, arrange a meeting with their representatives, especially the development leads. Ensure you’re accompanied by an experienced tech expert (such as a CTO) who can assess whether the potential vendor’s skills and resources align with your project needs.
Conclusion
If you’re looking for a trusted partner to develop custom medical device software, IntelliSoft is ready to help you! We have helped Cambio Healthcare Systems to develop and integrate innovative MedTech solutions since 2007. Our team is highly skilled in navigating the complexities of development, bringing deep expertise in areas like AI, IoT, and the all-important regulatory compliance required for medical devices.
At IntelliSoft, we understand that adhering to strict regulatory standards is non-negotiable in healthcare. That’s why we put compliance at the heart of everything, aligning our solutions with key industry regulations such as HIPAA and FDA medical device software development. This dedication ensures that our digital product isn’t just cutting-edge—it’s also fully compliant with the legal requirements that govern the healthcare sector.
But we don’t stop at delivering top-tier software. Our commitment extends to ongoing maintenance, updates, and support, ensuring that our solutions continue to lead the way in an ever-changing healthcare landscape. Contact us today for more information!